Contributing to Evidence-Based Regulatory Decisions: A Comparison
Por um escritor misterioso
Last updated 25 outubro 2024

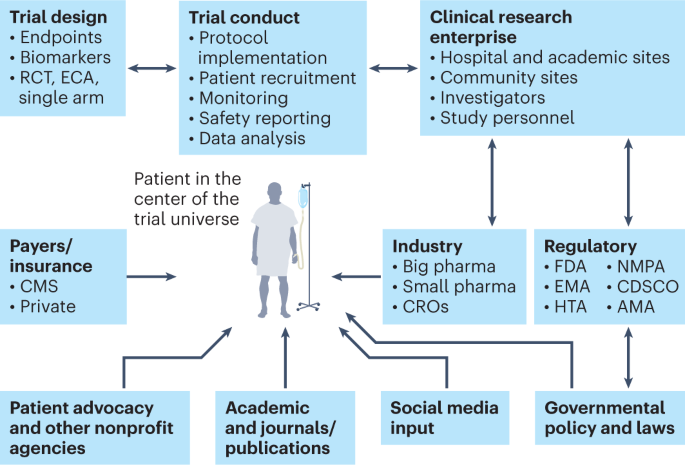
The next generation of evidence-based medicine

Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind

Make Better Decisions with Evidence-based Comparisons in Atlas, by Daniel Krawec, HumanFirst, Oct, 2023

NIH's Definition of a Clinical Trial

Randomized controlled trial - Wikipedia

Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis - The Lancet

20 Standardized Tests Pros And Cons (2023)
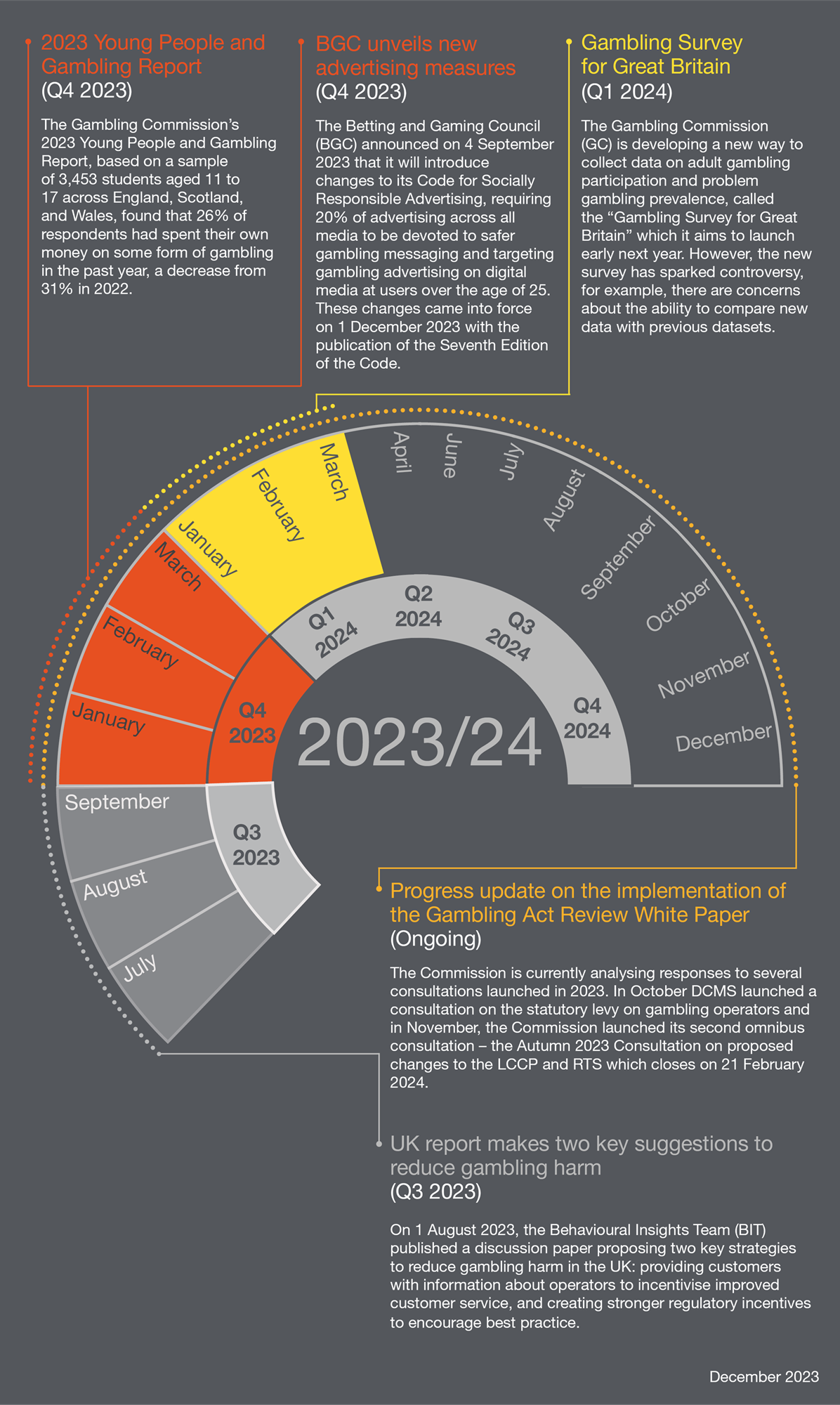
Betting and Gaming horizon scanning: UK regulatory roadmap: Winter 2023 edition - Lexology
The Value of an Optimized Clinical Data Strategy: How Small Changes Can Make a Big Difference - Cytel
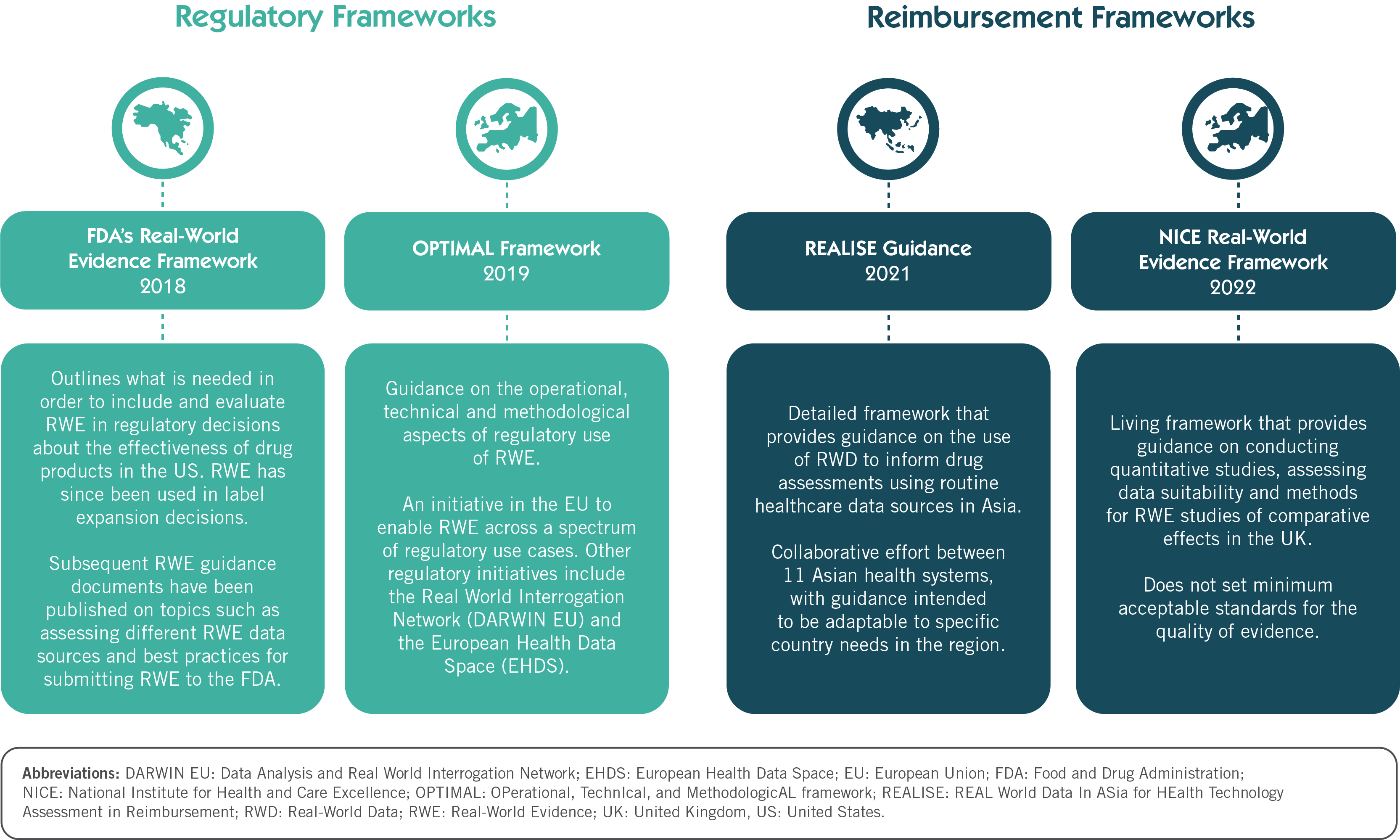
The Dawn of Real-World Evidence Frameworks: Where Does NICE Fit In?
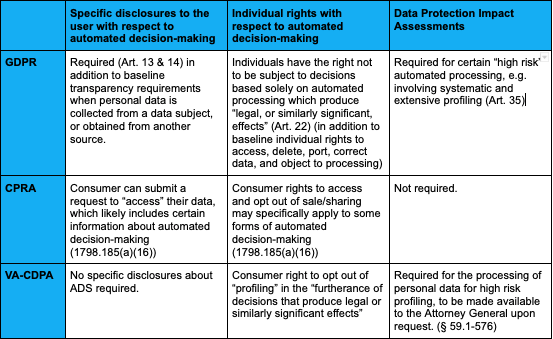
Automated-Decision Making Systems: Considerations for State Policymakers
Recomendado para você
-
 Banished Words Listed By Year 1976 - 202225 outubro 2024
Banished Words Listed By Year 1976 - 202225 outubro 2024 -
 What Does I Forgor 💀 Mean?25 outubro 2024
What Does I Forgor 💀 Mean?25 outubro 2024 -
Example entry on Urban Dictionary, including the head word (125 outubro 2024
-
 SARS-CoV-2 incidence, transmission, and reinfection in a rural and25 outubro 2024
SARS-CoV-2 incidence, transmission, and reinfection in a rural and25 outubro 2024 -
 What is securitization? Definition, process & consequences - TheStreet25 outubro 2024
What is securitization? Definition, process & consequences - TheStreet25 outubro 2024 -
 Femlandia25 outubro 2024
Femlandia25 outubro 2024 -
 Royal Building of Mafra – Palace, Basilica, Convent, Cerco Garden25 outubro 2024
Royal Building of Mafra – Palace, Basilica, Convent, Cerco Garden25 outubro 2024 -
 Money Slang: Decoding Financial Terms25 outubro 2024
Money Slang: Decoding Financial Terms25 outubro 2024 -
 Electrochemically Generated Interfacial pH Change: Application to25 outubro 2024
Electrochemically Generated Interfacial pH Change: Application to25 outubro 2024 -
 2021 ISHNE/HRS/EHRA/APHRS Expert Collaborative Statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society25 outubro 2024
2021 ISHNE/HRS/EHRA/APHRS Expert Collaborative Statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society25 outubro 2024
você pode gostar
-
 DLSS 3 Mods Released for Assassin's Creed Mirage and Lies of P25 outubro 2024
DLSS 3 Mods Released for Assassin's Creed Mirage and Lies of P25 outubro 2024 -
Top Tier Accounting ® (@toptieraccountingservices) • Instagram25 outubro 2024
-
 Nightmare Chica, Five Nights at Freddy's Wiki25 outubro 2024
Nightmare Chica, Five Nights at Freddy's Wiki25 outubro 2024 -
Metagame - STAAABmons25 outubro 2024
-
 Haikyu!!, Black Clover e mais: Confira os animes dublados da25 outubro 2024
Haikyu!!, Black Clover e mais: Confira os animes dublados da25 outubro 2024 -
 Next Week: Luffy's Peak - Attained! Gear Five25 outubro 2024
Next Week: Luffy's Peak - Attained! Gear Five25 outubro 2024 -
 Netflix Is Combining Its Two Sports Loves, and the PGA Tour Will Happily Draft Off It - Sports Illustrated Golf: News, Scores, Equipment, Instruction, Travel, Courses25 outubro 2024
Netflix Is Combining Its Two Sports Loves, and the PGA Tour Will Happily Draft Off It - Sports Illustrated Golf: News, Scores, Equipment, Instruction, Travel, Courses25 outubro 2024 -
 The Naked Monster - Wikipedia25 outubro 2024
The Naked Monster - Wikipedia25 outubro 2024 -
 Zagueiro revelado pelo Corinthians batê pênalti ousado e define título do Zenit; veja vídeo25 outubro 2024
Zagueiro revelado pelo Corinthians batê pênalti ousado e define título do Zenit; veja vídeo25 outubro 2024 -
format(webp)) Yowamushi Pedal Limit Break Anime Makes a Rest Stop for New Year's - Crunchyroll News25 outubro 2024
Yowamushi Pedal Limit Break Anime Makes a Rest Stop for New Year's - Crunchyroll News25 outubro 2024
