GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Last updated 22 dezembro 2024

GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.

Human growth hormone (32-38) Formula - C39H60N8O13 - Over 100 million chemical compounds

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g

A compound is found to contain 39.99% carbon, 6.727% hydrogen, and 53.28% oxygen by mass. The molar mass for this compound is 90.09 g/mol. What is the molecular formula for this compound?

SOLVED:What is the mass of the molecular ion formed from compounds having each molecular formula: (a) C3H6O; (b) C10H20; (c) C8H8O2; (d) methamphetamine (C10H15N)? How to use the mass of the molecular
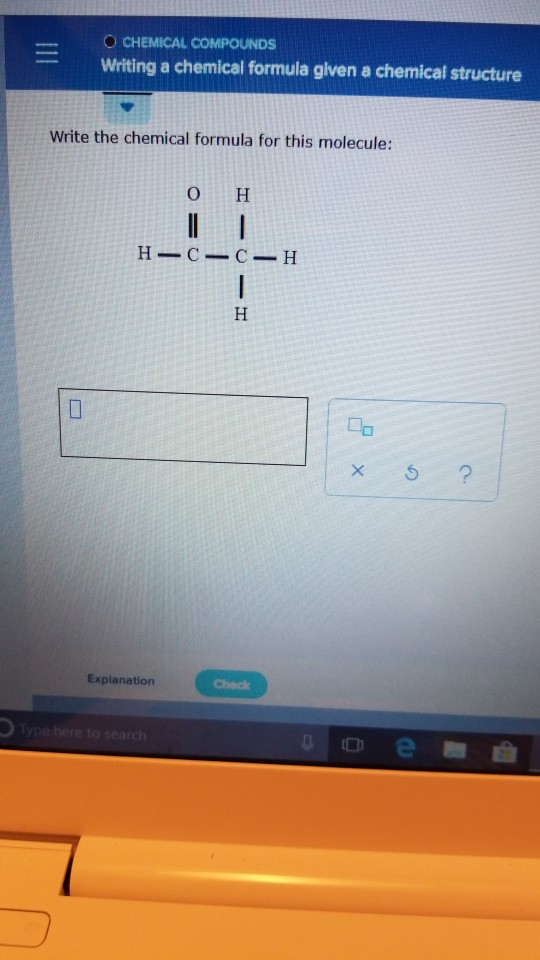
Solved O CHEMICAL COMPOUNDS Writing a chemical formula given
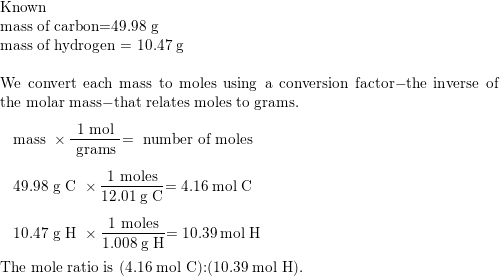
A compound was found to contain 49.98 g of carbon and 10.47

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
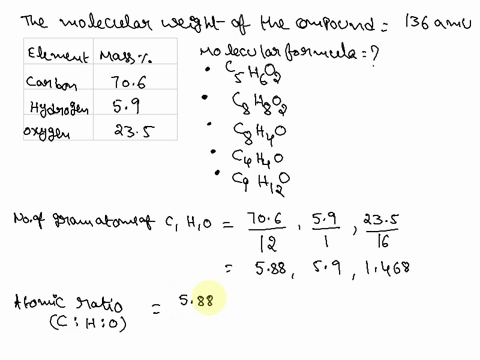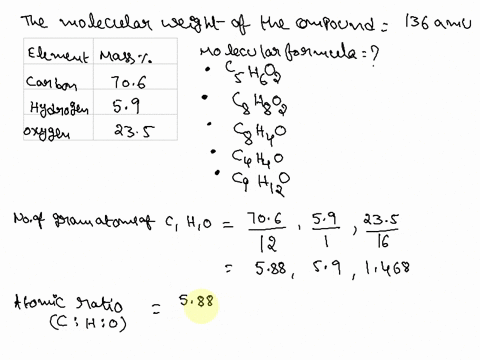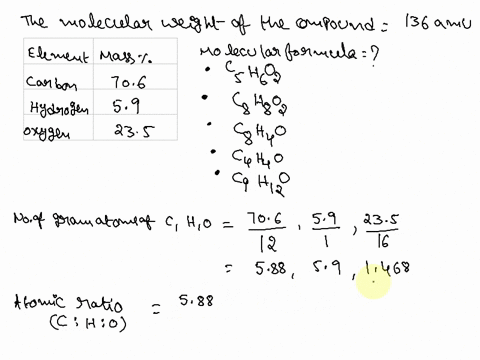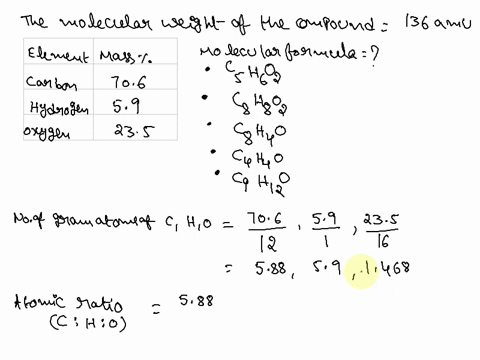
SOLVED: Question 15 (2 points) Determine the molecular formula for a compound that is 46.16% carbon; 5.16% hydrogen; and 48.68% fluorine: The molar mass of the compound is 156.12 grams. CaH4F CsH4F CsHF4 CaHF2
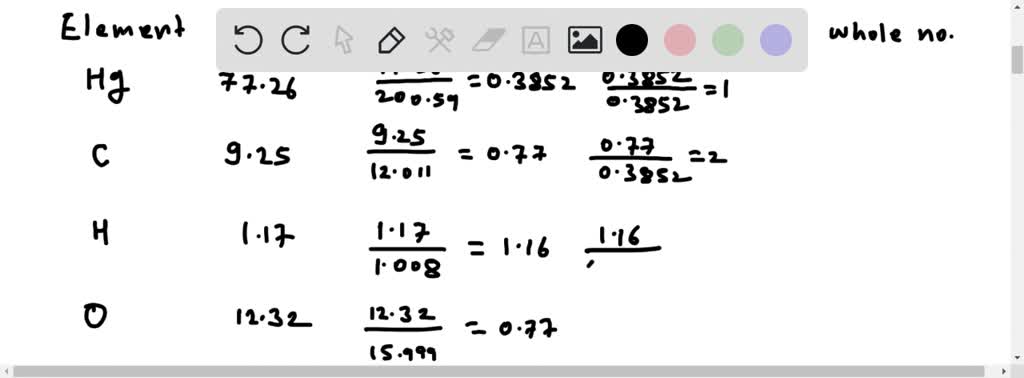
⏩SOLVED:One compound of mercury with a molar mass of 519 contains…
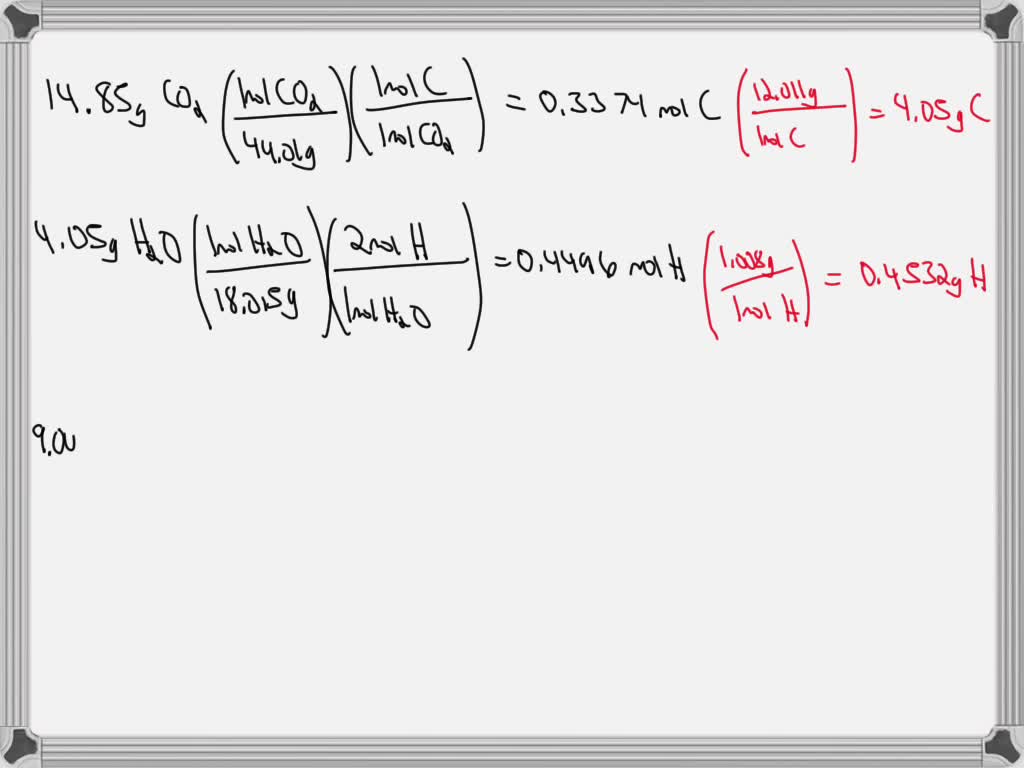
SOLVED: 9.00 g of a certain Compound X, known to be made of carbon, hydrogen, and perhaps oxygen, and to have a molecular molar mass of 152 g/mol, is burned completely in
Recomendado para você
-
 Tiverton GHGH GH COLOGNE FOR MEN 3.4 OZ / 100 ML EAU DE PARFUM SPRAY22 dezembro 2024
Tiverton GHGH GH COLOGNE FOR MEN 3.4 OZ / 100 ML EAU DE PARFUM SPRAY22 dezembro 2024 -
Steam Workshop::ghgh22 dezembro 2024
-
Ghgh - Ghgh added a new photo.22 dezembro 2024
-
 ghgh Minecraft Skins22 dezembro 2024
ghgh Minecraft Skins22 dezembro 2024 -
 1 Free Ghgh music playlists22 dezembro 2024
1 Free Ghgh music playlists22 dezembro 2024 -
 Urbeez La-reunion - Members - Search - Ghgh22 dezembro 2024
Urbeez La-reunion - Members - Search - Ghgh22 dezembro 2024 -
 F-GHGH, FGHGH, Most Recent Photos22 dezembro 2024
F-GHGH, FGHGH, Most Recent Photos22 dezembro 2024 -
rakka / ghgh piano fuzz22 dezembro 2024
-
 GHGH by COSMO DESIGNS22 dezembro 2024
GHGH by COSMO DESIGNS22 dezembro 2024 -
 F-GHGH - Boeing 767-37EER, Air France22 dezembro 2024
F-GHGH - Boeing 767-37EER, Air France22 dezembro 2024
você pode gostar
-
 Oppenheimer: conheça 5 filmes parecidos baseados em cientistas reais22 dezembro 2024
Oppenheimer: conheça 5 filmes parecidos baseados em cientistas reais22 dezembro 2024 -
 Update 1.0.7 Announcement and Patch Notes news - SCP - Containment Breach (Graphics Overhaul Mod) for SCP - Containment Breach - ModDB22 dezembro 2024
Update 1.0.7 Announcement and Patch Notes news - SCP - Containment Breach (Graphics Overhaul Mod) for SCP - Containment Breach - ModDB22 dezembro 2024 -
 Brasil x Argentina Horário, onde assistir, palpites e prováveis escalações22 dezembro 2024
Brasil x Argentina Horário, onde assistir, palpites e prováveis escalações22 dezembro 2024 -
 GTA: Online. • Customise the Nagasaki BF400. • Black and white. • At LS Customs. • 🔧🪛22 dezembro 2024
GTA: Online. • Customise the Nagasaki BF400. • Black and white. • At LS Customs. • 🔧🪛22 dezembro 2024 -
 Hajime No Ippo Complete Series Episodes 126 + Movie Champion Road22 dezembro 2024
Hajime No Ippo Complete Series Episodes 126 + Movie Champion Road22 dezembro 2024 -
 Outdoor Movie Night @ Elmaro Vineyards - Around River City22 dezembro 2024
Outdoor Movie Night @ Elmaro Vineyards - Around River City22 dezembro 2024 -
 Gaetano Castrovilli - Player profile 23/2422 dezembro 2024
Gaetano Castrovilli - Player profile 23/2422 dezembro 2024 -
 Banana Fish Vol. 2 : Yoshida, Akimi: : Livros22 dezembro 2024
Banana Fish Vol. 2 : Yoshida, Akimi: : Livros22 dezembro 2024 -
 Smeagol, Helpful Guide Deck for Magic: the Gathering22 dezembro 2024
Smeagol, Helpful Guide Deck for Magic: the Gathering22 dezembro 2024 -
 6 Fun-Filled Games to Play Outside with 3 Players Right Now22 dezembro 2024
6 Fun-Filled Games to Play Outside with 3 Players Right Now22 dezembro 2024


